
Given that breakthrough designation is widely considered a successful regulatory invention, it is surprising to hear executives use it as excuse for failure. Mr Lis also pointed to the speed with which breakthrough designees move through the agency’s approval chain, suggesting that it has led to errors in its submission package that caused to the FDA’s negative decision. “We didn’t think it was necessary to provide additional healthy volunteers,” chief executive William Lis said during the call. Specifically, regulators want to see additional dosing data in healthy volunteers who have been exposed to Lixiana and Lovenox – two agents that represent a small share of the market compared with Xarelto and Eliquis. However, a request for more clinical data has the potential to set back a resubmission rather longer. The bulk of the FDA’s complete response letter was around chemistry, manufacturing and controls, Portola executives said in a call with investors today, an issue that sometimes can be resolved in a short amount of time. In a sign that the group may have overreached, US regulators want more data on its use as an antidote to Lixiana and Lovenox, suggesting a re-submission could take months or longer.
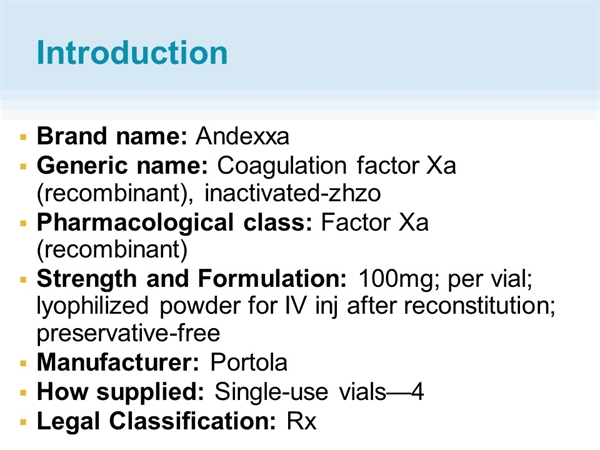
Two setbacks for Portola Pharmaceuticals in the space of five months have sent its share prices tumbling to levels not seen since it floated in 2013.Īndexxa’s failure to gain a green light from the US FDA is the bigger of the two blows, as the blood-thinner antidote had been widely expected to breeze through after receiving key pre-approval endorsement in the form of breakthrough therapy designation.


 0 kommentar(er)
0 kommentar(er)
